Abstract
INTRODUCTION
Age-related clonal hematopoiesis (ARCH), which was initially suggested by X-chromosome inactivation (XCI) studies, is associated with acquired mutations in driver and non-driver genes. ARCH is highly prevalent after the age of 65, and infers an increased risk of hematological cancers and cardiovascular complications. Quantification of these risks in individual subjects is not possible at this time, leading to the creation of a clinical entity named Clonal Hematopoiesis of Indeterminate Potential, or CHIP (Steensma et al., Blood 2015). There is a need to determine factors that modulate the oncogenic penetrance of these mutations. Identifying the origin within the hematopoietic stem cell (HSC) hierarchy of the mutations responsible for ARCH is a crucial initial step. We recently documented that 93% of driver mutations in ARCH occurred in DNMT3A or TET2 (Buscarlet et al., Blood. 2017). We now report the comparative hematopoietic lineage involvement of these two genes in a cohort of 86 individuals with ARCH.
METHODS
We have previously identified driver mutations in polymorphonuclear cells (PMN) of 347 individuals from a cohort of 2350 individuals. We have selected 86 individuals on the basis of having a single DNMT3A or TET2 mutation and the availability of DNA from PMN (granulocytes), CD14+ (monocytes), CD19+ (B-cells) and CD3+ (T-cells) allowing lineage involvement analysis. The lineage specific cells were obtained by Fluorescence-activated cell sorting (FACS) from the mononuclear cell fraction of peripheral blood samples. All samples were sequenced at high coverage (95% > 500x) on an Ion Proton sequencing using the AML Ampliseq panel. All mutations were visually inspected on Integrative Genomics Viewer (IGV). Samples were considered mutated if the mutation was detected above 2% VAF (variant allele frequency).
RESULTS
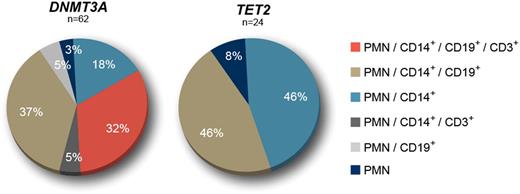
The study cohort comprised 86 individuals from 55 to 96 years-old (mean age: 69.0). Sixty two (62) had a single DNMT3A mutation (mean VAF in PMN = 11.2%) and 24 had a single TET2 mutation (mean VAF in PMN = 10.5%). TET2 mutated individuals where older than those with DNMT3A mutations (72.3 vs 67.7, P < .05). From the 62 DNMT3A mutations initially detected in PMN, 32.3% were present in all cell types analysed (PMN, CD14+, CD19+ and CD3+), 37.1% were detected in PMN, CD14+ and CD19+ cells, and 21.0% were found only in PMN and CD14+ cells. The remaining mutations were detected in PMN, CD14+ and CD3+ cells (4.8%), PMN and CD19+ cells (4.8%), or only in PMN (3.2%). For TET2, 45.8% of the mutations were detected in PMN and CD14+ cells, 45.8% were found in PMN, CD14+ and CD19+ cells, and 8.3% only detected in PMN. No mutations were detected in CD3+ cells. The different lineage involvements were statistically different between DNMT3A and TET2 for myeloid restriction (PMN or PMN and CD14+ cells): 21% (13/62) vs 54.2%, (13/24), P < .05; and for mutation in CD3+ cells: 37% (23/62) vs 0% (0/24), P < .001. Although the proportion of subjects with myeloid and B-cell involvement was similar between the two genes, the CD14+/CD19+ VAF ratio was significantly higher for TET2 (3.13, n = 11) than for DNMT3A (1.94, n = 43) (P < .005). Selecting subjects who had a VAF > 10% in PMN (suggesting a longer evolution) had no impact on the myeloid lineage restriction for TET2, but almost all (21/22) DNMT3A samples showed mutations in both myeloid and lymphoid compartments.
CONCLUSION
These results demonstrate a significantly different lineage involvement between DNMT3A and TET2 mutations in ARCH. DNMT3A mutations predominantly affect both the myeloid and lymphoid compartment, including T-cells in a third of subjects. This strongly suggests that DNMT3A mutations occur in a HSC with multipotent proliferative potential. In contrast, we never documented TET2 mutations in T-cells, suggesting the involvement of more committed HSC. This is paradoxical since both DNMT3A and TET2 mutations have been documented in T-cell lymphoma. This could be explained by a different HSC origin for TET2 mutation in myeloid malignancies vs T-cell lymphomas. Alternatively, it could be that in normal aging individuals, the multipotent mutated HSC has a stronger myeloid proliferation bias than DNMT3A . This bias may be in response to homeostatic stimuli as we have documented a slight neutropenia in subjects with ARCH and TET2 mutations (Buscarlet et al., Blood 2017).
Busque: Bristol Myer Squibb: Honoraria; Novartis Canada Inc.: Honoraria; Paladin: Honoraria; Pfizer: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal